
The „perfect Market Access“ is a metaphor for a fast and all-encompassing access of patients to important health technologies, innovative therapies and treatment opportunities. It is a strategic imperative to communicate the right data and information to the right customer at the right time.
WHAT WE CAN DO FOR YOUR PRODUCTS:
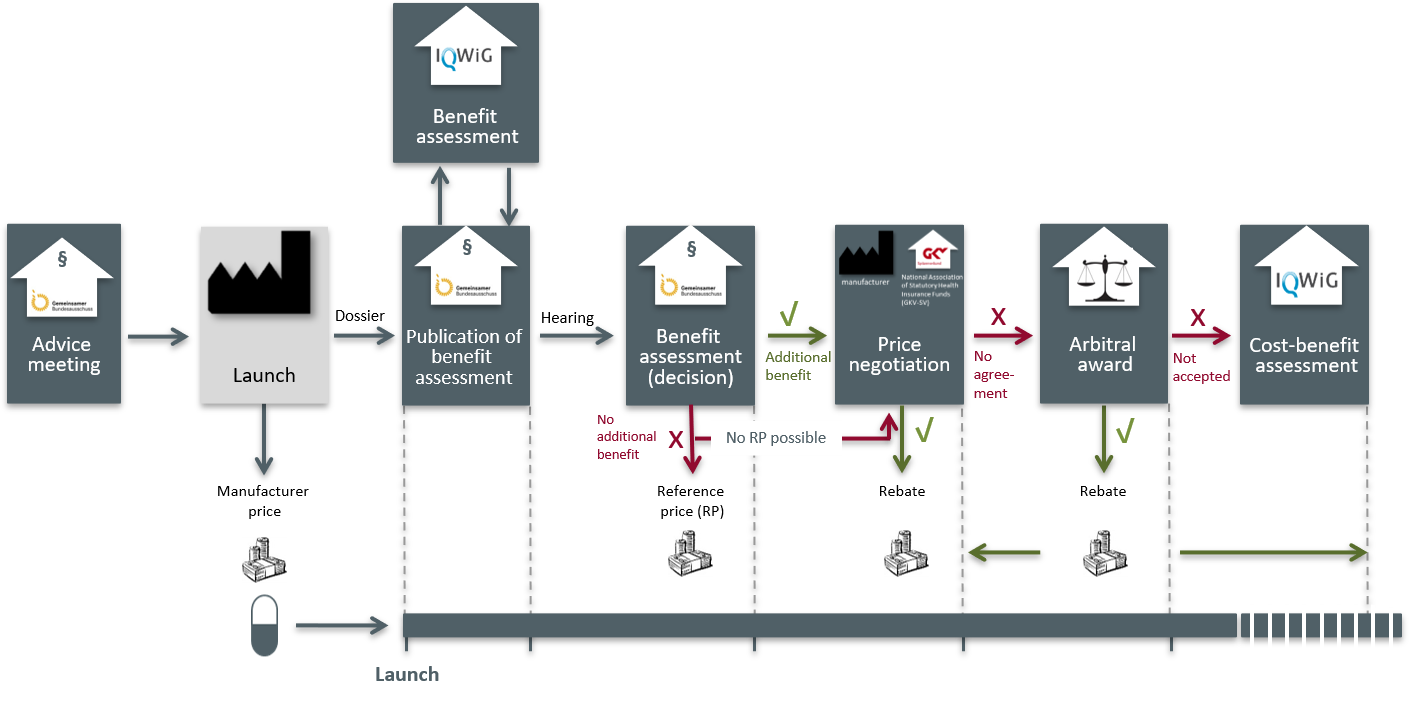
AMNOG is the German interpretation of the global megatrend for more evidence in reimbursement decisions of health technologies (Health Technology Assessment (HTA)). The support of Market Access is substantially more than ‘just’ AMNOG: Reimbursement of new products inpatient and outpatient, e.g. by NUB-applications, DRG workshops, applications for OPS codes, preparation of internationally harmonized/coordinated Market Access strategies, analysis of reference pricing, contracting models with payers and insurance companies, development and performance of ‘Sounding Boards’ for fruitful discussions with decision makers as well as interviews with selected stakeholders. For the upcoming negotiations during the Market Access process, we provide you with perfectly matching contacts and offer customized training, as well as our support during the process of negotiations and decision making. The monitoring and evaluation of results and measures is a matter of course for SmartStep.
MOTIVES & TOOLS:

- Faster access
➢ Recognition of added therapeutic benefit - Better reimbursement terms
➢ Better price and reimbursement negotiation results - Broader customer base
➢ Better value arguments communicated better - Better long-term results
➢ Lifecycle-management / contract strategy / patient management pathways /
compliance management
EXAMPLES OF SUCCESSFUL MARKET ACCESS PROJECTS:
- Development of overall strategy in European reimbursement incl. AMNOG, NICE, SMC, AIFA etc.
- Continuous monitoring of all AMNOG decisions from a methodological and result-focused point of view
- Development and evaluation of the consultation requests in oncology to the Federal Joint Committee (G-BA)
- Exemption/Liberation of AMNOG-dossier for hospital products
- Compilation of dossiers for orphan drugs and oncology products
- Stress tests of the requests for consultation and for dossiers
- Development of ‘objection handling’ and ‘Q&A documents’
- Development and support of written statements and oral hearings
- Risk analysis of the questioning/inspection of the established drug market in expectation of new price negotiations
- Implementation of trainings for oral hearings and statements
The earlier we start the better… Because the increasing uncertainty and complexity in research and development programs and investments, new regulatory strategies and the more complex marketing strategies require the early integration of Market Access activities into the product development process.
