In November 2019, the adoption of the Digital Health Care Act (DVG) made it possible for physicians and psychotherapists to prescribe digital health applications (DiGA). As a result of the expansion of the SGB V to include Section 33a (digital health applications), which is attributable to the DVG, DiGA became reimbursable by the statutory health insurance funds for the first time. The application and evaluation process is carried out by the Federal Institute for Drugs and Medical Devices (BfArM). The BfArM has three months to decide on applications from manufacturers. The application can refer to a final or provisional listing in the DiGA directory. Final DiGA status is granted to the manufacturers who have already provided full evidence of positive healthcare effects from the use of their DiGA. Provisionally listed DiGAs are expected to provide the required evidence within a one-year trial period.
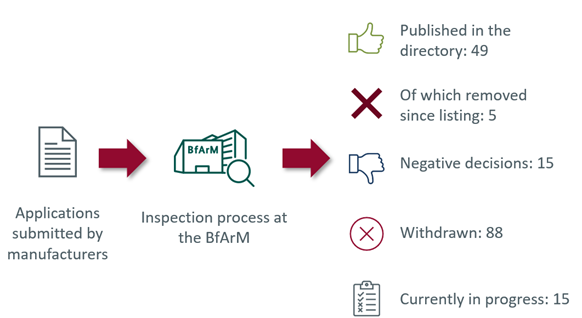
Of the total of 167 applications received by the BfArM, 49 DiGAs were listed in the directory (as of 2023-03-14). Five of these 49 DiGAs have already been removed from the list. In addition, the possibility of extending the trial phase was used for various DiGAs. This is particularly relevant in terms of pricing, as compensation amounts must be paid to the health insurance fund due to removal from the directory after the extended trial phase. For example, due to a lack of proven medical benefit, in one case the arbitration board set a compensation amount of ten euros for the period of the extended trial phase.
Proof of evidence and comparable healthcare context as major influence factors on DiGA reimbursement amount.
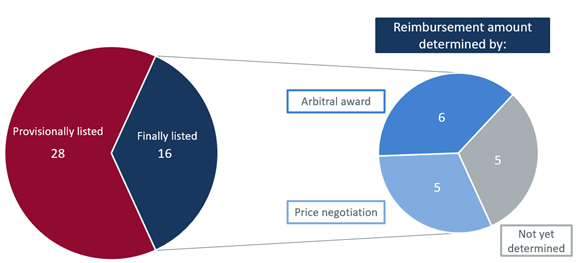
Of the eleven reimbursement amounts set to date, five were determined during price negotiations with the GKV-SV and six by the arbitration board chaired by Prof. Dr. Jürgen Wasem.
From the arbitration proceedings to date on the pricing of digital health applications, a systematic of the arbitration board is emerging which corresponds in essential parts to the AMNOG system for setting medicinal product prices. Following previous arbitration board decisions, the DiGA reimbursement amount is made up of the demonstrable positive healthcare effects (pVE) and the self-payer or European prices for the DiGA. The monetization of the pVE is done by deriving the comparable healthcare context (reimbursement level of health insurance funds) and a surcharge. However, the surcharge is not to be considered an “added benefit” in the sense of Section 35a SGB V, but rather an “effect size”.
It is apparent that, from the point of view of the arbitration board, the comparable healthcare context in particular serves as a relevant price anchor. According to the framework agreement under Section 134 (4) and (5), other price-relevant documents can also be submitted. These must be announced before the start of the first round of price negotiations and submitted to the other party at the latest before the second round of price negotiations. This supplementary information has been considered in previous arbitration awards to quantify the comparable healthcare context. For this purpose, health insurers’ healthcare data analyses were cited in four of the six arbitration proceedings. Other price-relevant documents submitted by the parties, such as health economic models or expert reports, have not yet been explicitly considered.
4 out of 6 of the previous arbitration decisions on the determination of the reimbursement amount referred to DiGAs in the indication group “psyche”. In these, cognitive behavioral therapy was used as a comparable healthcare context. For reasons of economic efficiency and considering the view of the arbitration board that DiGA cannot replace psychotherapy, treatment costs in the context of group therapy were assumed.
The arbitration board deviated from this approach in the recently published arbitral award on Vivira in the therapeutic area of back pain. Since the DiGA was able to show a significant reduction in pain after 12 weeks, as measured by the Verbal Numerical Rating Scale, compared to physiotherapy, the arbitration board assumed that Vivira could be an alternative to physiotherapy. As a result, physiotherapy in individual treatment was used as a comparable healthcare context.
The arbitration board’s considerations for determining the surcharge include, among others, study quality, benefit dimension, and magnitude of effect.
In addition, other factors are sometimes considered in the arbitral awards:
- Effect size of the comparator therapy
- Epidemiology of the disease
- Choice of comparator
- Potential for bias in the study/studies
Of the finally listed DiGAs, five DiGAs are currently in reimbursement negotiations (see Figure 2). In the event of one or more arbitration award(s), new information on considerations of the comparable healthcare context could follow and potentially provide new insight into the DiGA arbitration board approach.
„Make your next step smart”
SmartStep supports manufacturers of digital health applications throughout the entire DiGA process: starting with strategic analyses of the product idea and evidence generation through to the process of price negotiation. Experience shows that careful strategic considerations during the planning of evidence generation set the foundation for later negotiation success.
