Strategy, dossier, and statistics from a single source (accelerate the processes)
Most clinical trials are planned with the aim of approval of drugs by the FDA and EMA. Once the approval has been granted, the next hurdle is the reimbursement process. This raises not only the question of safety and efficacy of the substance in an indication area, , but also the question of an added benefit compared to the already approved and available drugs. This added benefit can be expressed, for example, in the form of improved efficacy (e.g. reduced symptom burden), longer survival and lower mortality, lower side effects or increased quality of life.
In Germany, G-BA and IQWiG place very specific and high demands on the study evaluations for the added benefit assessment, so almost every study needs to be re-evaluated based on these requirements. In addition, the label is often different from the study population and thus all analyses must be specifically carried out for subpopulations. To be able to investigate potential for improvement compared to the current German reality of care, patients with certain comparator therapies must also be included or excluded to allow for the transferability of study results in Germany.
Overall, the analyses defined in the study protocol and statistical analysis plan are not only necessary for the benefit assessment process, but also for special analyses required by the G-BA. In addition, post-hoc exploratory analyses can be implemented using the available evidence to answer questions regarding the benefit assessment process.
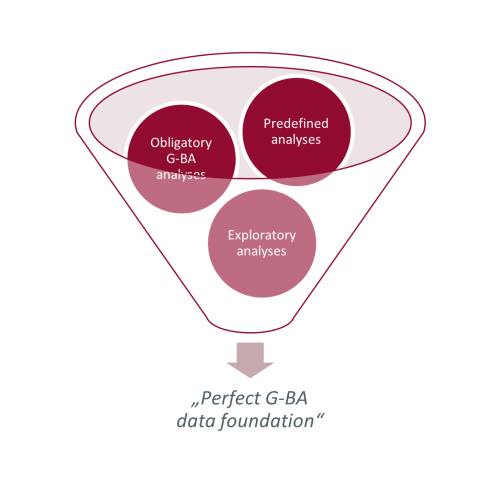
Figure 1: Building blocks of a data analysis for the benefit assessment in Germany
This requires a complex GAP analysis and a high degree of international communication between market access, medicals, and the evaluating statisticians. New evaluations and coordination are usually not planned in advance and involve a lot of effort and time pressure for all participants.
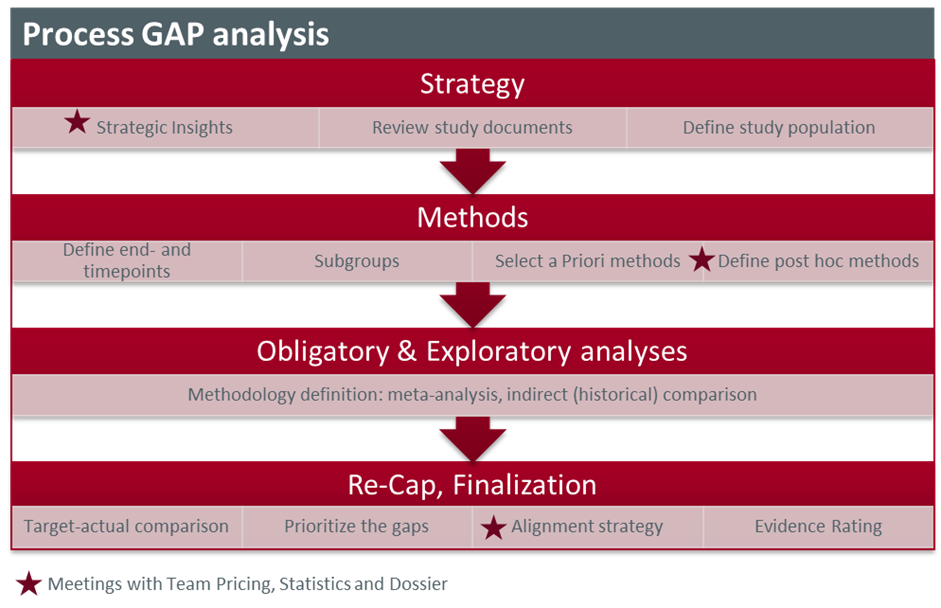
Figure 2: GAP-analysis: Preparation of data analysis
The strategy of the entire reimbursement process must first be defined before determining the scope of the necessary analyses. The current and future market situation is examined as a part of strategic analysis, and the current label and its effects are discussed. The study situation in the indication area must also be examined closely to determine whether there is direct comparative evidence or if an (adjusted) indirect comparison has been carried out. Can evidence from several studies be combined as part of a meta-analysis to achieve a higher level of evidence? Have signs of further benefit or harm been discovered as part of the FDA and EMA procedures? Are there any specific questions and requirements for ensuring that the data can be transferred to the German market? The methods for the following analyses are defined based on these investigations. Subsequently, the necessary analyses are prioritized and compared with the overall strategy, and then communicated with the evaluating statisticians. Firstly, the statisticians potentially carry out the analyses defined in SAP with populations adapted to the label, followed by the necessary analyses required for the German process. Exploratory analyses often involve the combination of several studies, as in the case of meta-analyses or indirect comparisons, or the definition of new endpoints based on data already collected in the studies.

Figure 3: Process of data analysis
Complete support of the HTA process from a single source
Due to the complexity of the process and the necessary close integration of the specialists involved in the project, SmartStep Consulting decided more than 5 years ago to establish an optimized strategy to complete the evaluation for the entire HTA process. This includes the strategy, dossier preparation, opinion submission, hearing preparation, and possible subsequent submissions including price negotiation. In addition to the analysis and registries of clinical trials, we also regularly carry out (network) meta-analyses, indirect comparisons, as well as fixed-amount simulations. The scope of the projects we carry out ranges from the effect estimation of a few endpoints to the evaluation of pivotal studies, analysis of several studies in various subpopulations with indirect comparisons, meta-analyses, and analyses of data for prevalence estimation.
Our statistics department has many years of experience in the AMNOG context and works in close collaboration with colleagues in the areas of strategy, medical writing, and pricing. For example, customized output tables decisively reduced the effort and error-proneness in transferring the results into the dossier, and close coordination between the teams enabled the complete analyses of several studies with a new population to be performed and prepared in time for the written statement.
Figure 4: Composition of the project team
The entire benefit assessment process can be made flexible and efficient by eliminating the need for coordination between different teams, coordinated timelines, one-stop market access, and short returns on time-critical analyses for comments and hearings.
Do you have any questions?
Simply contact us by e-mail and we will be happy to advise you.
