Background
The early benefit assessment of drugs according to §35a SGB V requires the demonstration of the clinical relevance of treatment effects, which essentially means determining the point at which a change becomes noticeable for the patient. This approach aims to enhance result interpretability and aid in deriving treatment recommendations.
Different concepts exist in the literature to distinguish patients with and without a minimal clinically important difference (MCID) on a point scale for a specific outcome (e.g., improvement in quality of life measured by the SF 36 questionnaire).
Based on the observed variability and the resulting range of reported MCID thresholds, including those for the same measurement instrument, the IQWiG introduced a new approach to demonstrate clinical relevance in the early benefit assessment. This was accomplished through the introduction of the methods paper V6.0, which was based on an analysis of the available literature (refer to the figure below). The decision to incorporate this approach into the module template in Annex II to Chapter 5, amending the Rules of Procedure, was made on December 16, 2021, and it officially came into effect on March 23, 2022.

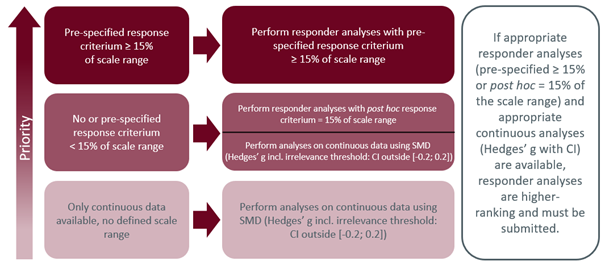
IQWiG defines a regular threshold (at least 15% of the scale range of the measurement instrument used or exactly 15% of the scale range in the context of a post hoc defined responder analysis) as the preferred alternative to the presentation of standardized mean differences in order to represent a change noticeable to patients with sufficient certainty. In contrast, thresholds validated for a specific indication and possibly already established in clinical practice, but do not meet these criteria, are generally not considered.
One-Size-Fits-All?
Experts from science, practice, and representatives of the pharmaceutical industry critically evaluate this “one-size-fits-all” approach, and there are good reasons for it.
A regular threshold for demonstrating clinical relevance is not only inconsistent with the complex relationship between MCID and numerous contextual factors, such as the characteristics of the patient population, the duration of the observation period, the direction of effect, and the dimension of benefit studied but also makes it difficult to place results in the context of prior benefit assessment procedures. A report by the ISPOR Clinical Outcome Assessment Special Interest Group finds that the so-called 15% threshold is often many times larger than its clinical data-based counterpart ((Is IQWiG’s 15% Threshold Universally Applicable in Assessing the Clinical Relevance of Patient-Reported Outcomes Changes? An ISPOR Special Interest Group Report – Value in Health (valueinhealthjournal.com)). The authors conclude that, compared to previous HTA decisions, the new regulation no longer focuses on the individual patient and replaces the patient-centered approach with a technical approach.
Changes measured in natural units or using (at least theoretically) unlimited scales (e.g., 6-minute walk test) are not covered by the new regulation and still require a validated threshold based on clinical data. Numerous exceptions (e.g., for the SF 36 questionnaire to measure the quality of life) also contribute to methodological diversity and thus complexity in the evaluation.
Be prepared – make your next step smart!
In the context of the upcoming European assessment (EU HTA) to be integrated into the German system, the fixed 15% threshold currently plays no role., Instead, to ensure the interpretability of results, the EUnetHTA 21 Individual Practical Guideline Document D4.4 – Outcomes (EUnetHTA-21-D4.4-practical-guideline-on-Endpoints-v1.0.pdf)) calls for the classification of patients who do or do not show a noticeable treatment effect in the context of responder analysis and using a justified threshold.
SmartStep pursues and accompanies the current developments of national and international methodological recommendations, offering its clients goal-oriented and strategic consulting for method-compliant evaluation and presentation of clinical results in the context of the AMNOG and the upcoming EU HTA.
Do you have any questions?
Simply contact us by e-mail and we will be happy to advise you!
